Establishing the functional connectivity in the visual cortex
Khdour H.Y., Kondabolu K., Khadka A., Assous M., Tepper J., Tran T., and Polack P-O. (2022) Neuropilin 2/Plexin-A3 Receptors Regulate the Functional Connectivity and the Excitability in the Layers 4 and 5 of the Cerebral Cortex. The Journal of Neuroscience. 42(24):4828–4840
The functions of cortical networks are progressively established during development by series of events shaping the neuronal connectivity. Synaptic elimination, which consists of removing the supernumerary connections generated during the earlier stages of cortical development, is one of the latest stages in neuronal network maturation. The semaphorin 3F coreceptors neuropilin 2 (Nrp2) and plexin-A3 (PlxnA3) may play an important role in the functional maturation of the cerebral cortex by regulating the excess dendritic spines on cortical excitatory neurons. Yet, the identity of the connections eliminated under the control of Nrp2/PlxnA3 signaling is debated, and the importance of this synaptic refinement for cortical functions remains poorly understood. Here, we show that Nrp2/PlxnA3 controls the spine densities in layer 4 (L4) and on the apical dendrite of L5 neurons of the sensory and motor cortices. Using a combination of neuroanatomical, ex vivo electrophysiology, and in vivo functional imaging techniques in Nrp2 and PlxnA3 KO mice of both sexes, we disprove the hypothesis that Nrp2/PlxnA3 signaling is required to maintain the ectopic thalamocortical connections observed during embryonic development. We also show that the absence of Nrp2/PlxnA3 signaling leads to the hyperexcitability and excessive synchronization of the neuronal activity in L5 and L4 neuronal networks, suggesting that this system could participate in the refinement of the recurrent corticocortical connectivity in those layers. Altogether, our results argue for a role of semaphorin–Nrp2/PlxnA3 signaling in the proper maturation and functional connectivity of the cerebral cortex, likely by controlling the refinement of recurrent corticocortical connections.
Figure
Caption
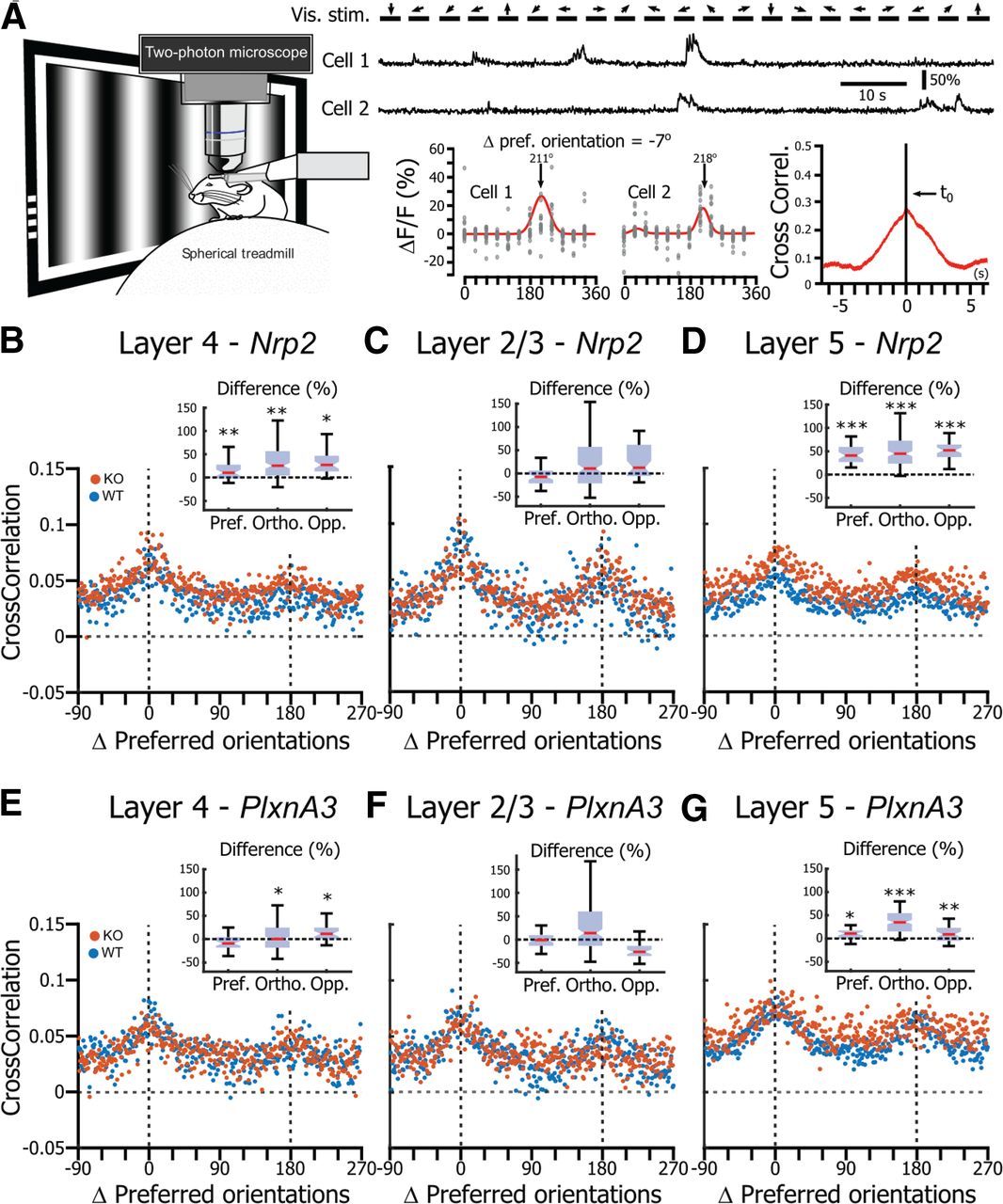
Neuronal activity correlation in V1 in the absence of Nrp2/PlxnA3 signaling. A, Left, Schematic representation of the correlation analysis. Top, Example of two neurons recorded simultaneously while drifting gratings (arrows; 6 orientations, 2 directions) were presented on the screen. Bottom left, Orientation tuning curves of the two neurons shown above. Bottom right, Cross-correlogram of the neuronal activity of the two neurons recorded during the presentation of visual stimuli. B, Correlation as a function of the distance (Δ) between the preferred orientations of the neuronal pairs for neurons recorded in L4 of Nrp2 WT (blue) and Nrp2 KO (orange) mice. Inset, Difference between the correlation measured in KO compared with WT when the neurons have similar pref. orientations, ortho. preferred orientations, or opp. preferred orientations. Asterisks indicate the effect size: *small effect size, **medium effect size, ***large effect size. C, Same representation as in B for L2/3 Nrp2 WT and Nrp2 KO neurons. D, Same representation as in B for L5 Nrp2 WT and Nrp2 KO neurons. E, Same representation as in B for L4 PlxnA3 WT and PlxnA3 KO neurons. F, Same representation as in C for L2/3 PlxnA3 WT and PlxnA3 KO neurons. G, Same representation as in D for L5 PlxnA3 WT and PlxnA3 KO neurons.